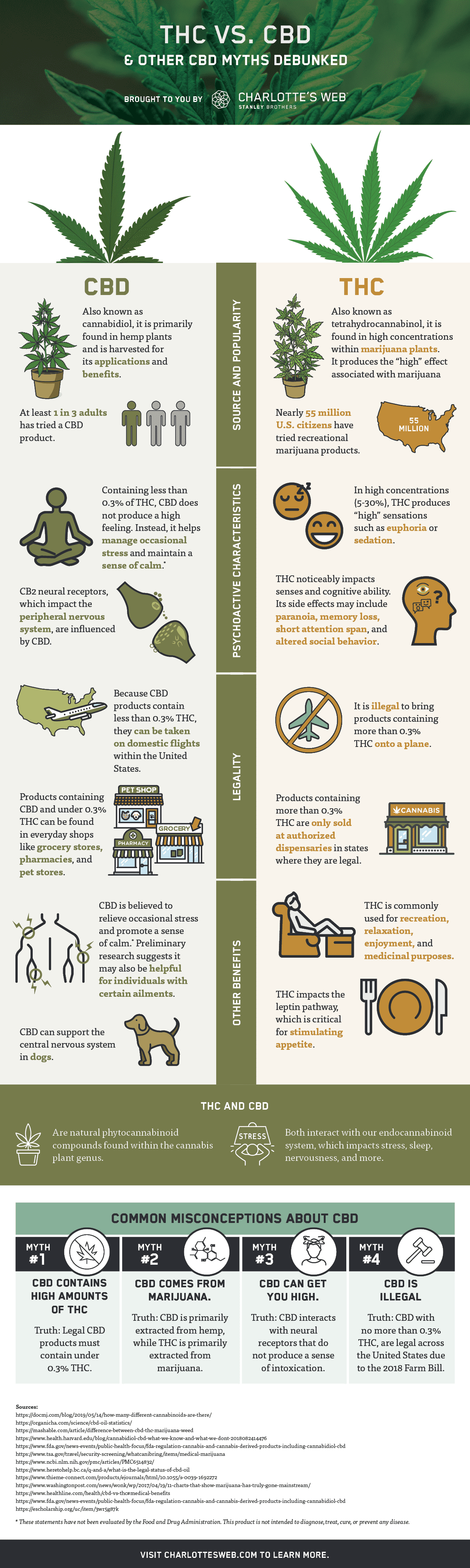
The Facts About Does Cbd Get You High? – Goodrx Uncovered
If a product is intended to affect the framework or feature of the body, or to detect, treat, alleviate, deal with or avoid illness, it is a drug, or perhaps both a cosmetic and a medication, even if it influences the appearance. (See Inquiry # 3 for more details about drugs.) FDA can act if it knows that a component or aesthetic product is unsafe to consumers.
Those aspects include, to name a few points, firm sources and also the risk to the public health and wellness. FDA likewise may talk to its government as well as state companions in making choices about whether to launch a government enforcement action. A. General details about the import/export of medication items controlled by FDA can be discovered online here.
as well as, thus, ought to be sought advice from respect to any type of regulations/requirements they may have pertaining to the import or export of products containing cannabis. Please see right here for information regarding importing or exporting food ingredients. Regarding imports, if it shows up that a post is faulty, misbranded, in infraction of area 505 of the FD&C Act, or prohibited from introduction or delivery for intro into interstate commerce under section 301(ll) of the FD&C Act, such post will be rejected admission (see section 801(a)( 3) of the FD&C Act [21 U.S.C.
6 Simple Techniques For What Is Cbd? This Cannabis Ingredient Is On The Rise For …
A. To conduct clinical study that can lead to an approved new medicine, consisting of research study making use of products from plants such as cannabis, researchers require to work with the FDA and also send an IND application to the Center for Medicine Analysis as well as Study (CDER). The IND application procedure gives researchers a path to comply with that consists of regular interactions with the FDA to support reliable medication development while safeguarding the clients that are enrolled in the trials.
https://wayofleaf.com/blog/how-to-maximize-the-effects
This includes: an enrollment provided by the DEA; acquiring the marijuana for study from NIDA, within the National Institutes of Wellness, or another DEA-registered source; and evaluation by the FDA of the IND or INAD application and also research study procedure. Furthermore: For a Schedule I controlled compound under the CSA, DEA offers scientists with private investigator and procedure registrations as well as has Schedule I-level safety demands at the site cannabis will be studied.
What Is The Difference Between Cannabis Products And Cbd … for Dummies
Based on the results gotten in research studies conducted at the IND stage, sponsors may submit an advertising and marketing application for official approval of the medicine. A. No. The FDA believes that medically legitimate research study carried out under an IND application is the ideal method to determine what people could gain from using medications originated from marijuana.
Companies that develop medications and biologics, also called enrollers, can offer details regarding whether their drug/biologic is taken into consideration an eligible investigational drug under RTT as well as if they are able to supply the drug/biologic under the RTT Act. A. We recognize that moms and dads are trying to locate treatments for their children’s medical problems.
Caregivers and people can be confident that FDA-approved drugs have been meticulously examined for security, efficiency, and top quality, and are checked by the FDA once they are on the market. The FDA remains to sustain audio, scientifically-based research study right into the medical usages of medicine items consisting of cannabis or cannabis-derived substances, and will certainly remain to work with business fascinated in bringing safe, reliable, and top quality products to market.
The Only Guide to Cbd & Diabetes – Ada
The American University of Obstetricians as well as Gynecologists (ACOG) suggests that women that are pregnant or considering pregnancy must be motivated to cease marijuana use. In addition, ACOG keeps in mind that there are insufficient information to review the results of cannabis use on breastfed infants; as a result, marijuana usage is inhibited when nursing.
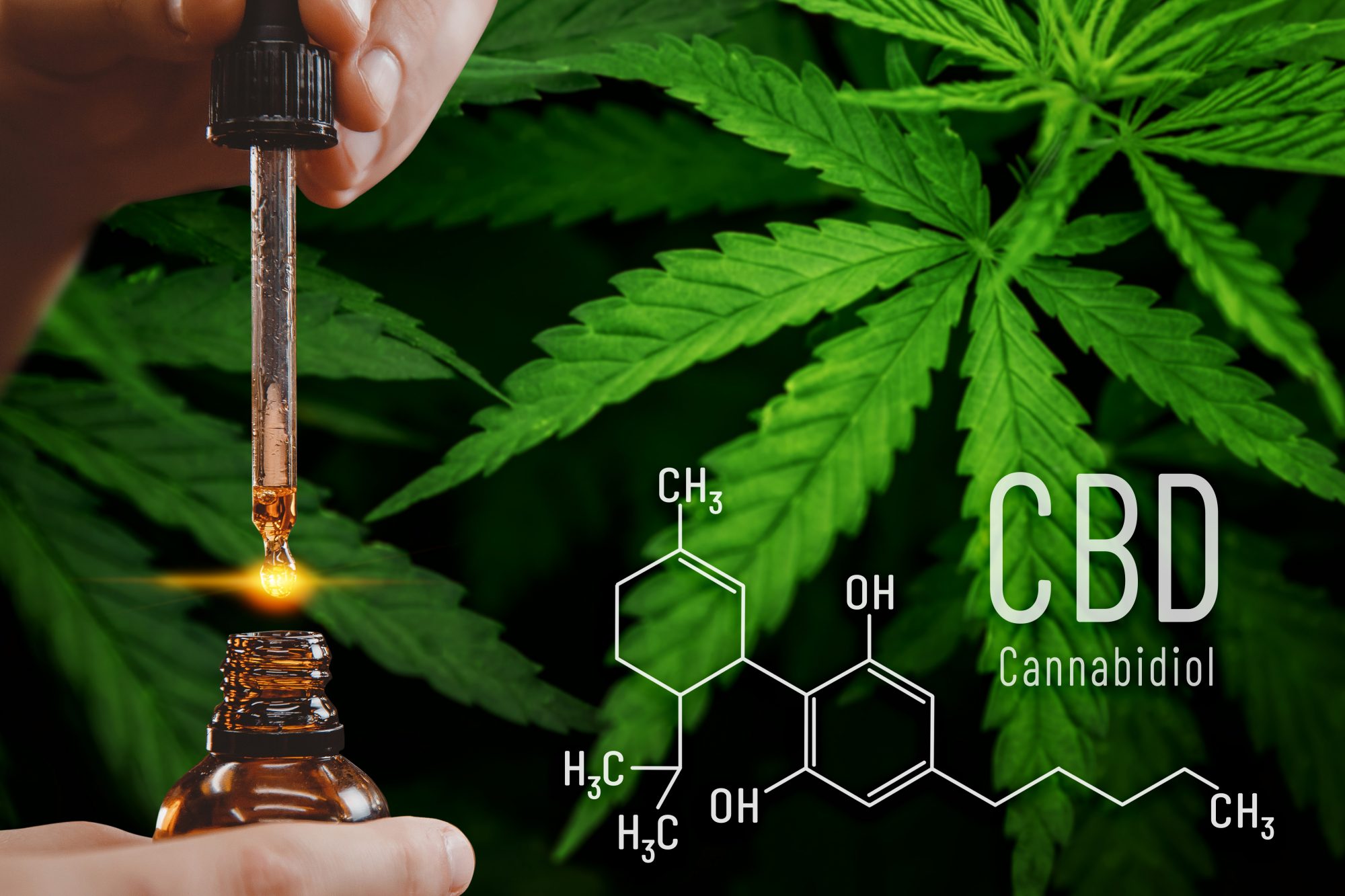
A. The FDA has actually accepted Epidiolex, which consists of a purified type of the drug material CBD, for the therapy of seizures linked with Lennox-Gastaut syndrome or Dravet disorder in patients 1 years old as well as older. It has additionally accepted Epidiolex for the therapy of seizures related to tuberous sclerosis facility in patients 1 year old or older.
Controlled clinical tests evaluating the safety and security and also efficacy of a medicine, in addition to cautious testimonial through the FDA’s drug authorization process, is the most suitable method to bring cannabis-derived therapies to patients. As a result of the adequate as well as well-controlled scientific research studies that sustained this authorization, and also the guarantee of making quality criteria, prescribers can have confidence in the drug’s consistent toughness and also consistent delivery that support suitable dosing required for dealing with patients with these facility as well as significant epilepsy syndromes.
The 10-Minute Rule for Edibles With Cbd May Bake In A Stronger High, Johns Hopkins …
With the exception of products such as the hemp seed ingredients reviewed in Inquiry # 12, which have actually been reviewed for security, it is essential to shield children from accidental intake of marijuana and also cannabis-containing items. FDA suggests that these products are shut out of reach of youngsters to minimize the risk of unintended intake.